
Doctors and scientists started the “Randomized Trial of Sedative Choice for Intubation (RSI)” in April of 2022. This website provides information about the RSI study.
The goal of the RSI trial is to learn which of two drugs (“ketamine” or “etomidate”) is best for patients receiving a breathing tube – overall and for specific groups of patients with medical problems like heart disease or severe infection.
Why the RSI Study is Needed:
Each year millions of seriously ill adults need life-saving treatment with a breathing machine. To safely place someone on a breathing machine (a procedure known as “intubation”), doctors give a drug to make the patient sleepy (known as a “sedative”). The two “sedative” drugs given most often to seriously ill patients receiving a breathing tube in the United States are ketamine and etomidate. Both drugs are approved by the United States Food and Drug Administration (FDA).
Both are considered safe and effective. Both are given by doctors all the time. But it is not known which drug is best.
Each drug may have benefits or risks. Ketamine may have a temporary negative effect on heart function in some patients. Etomidate may have a temporary negative effect on the hormones that help keep blood pressure normal. Early studies suggested that patients did equally well with the two drugs. Knowing for certain whether the two drugs really are the same for patients, or if one is better, could save many patients lives in the future.
What we are doing
Doctors and scientists are doing the “Randomized Trial of Sedative Choice for Intubation (RSI)” study to learn for certain whether ketamine and etomidate are the same for patients being placed on a breathing machine, or if one is better for preventing serious problems with blood pressure, oxygen levels, or heart function.
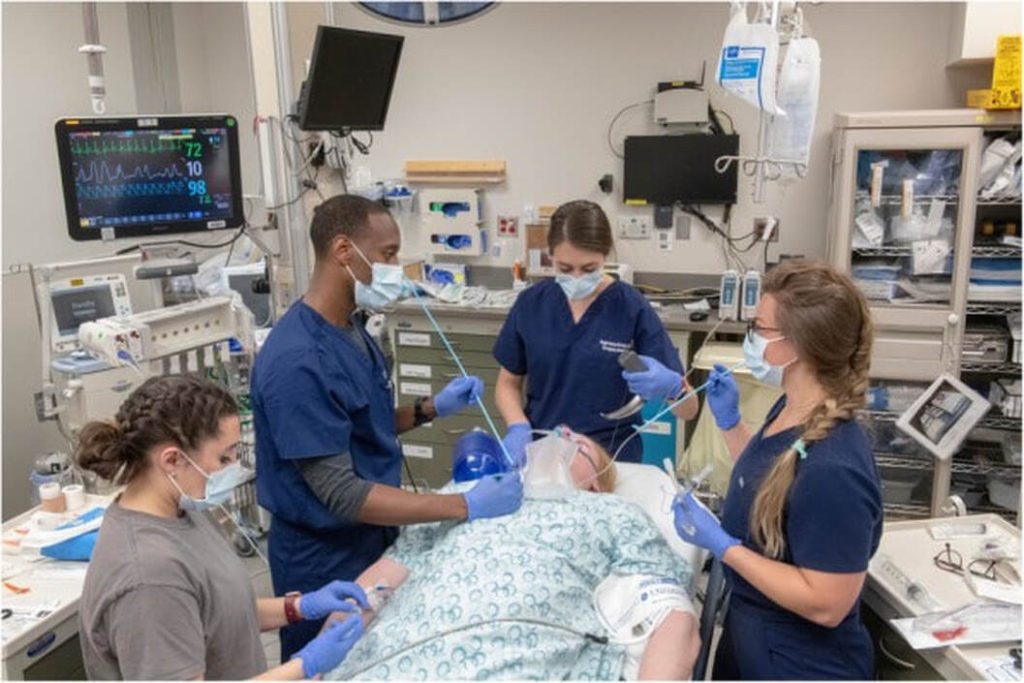
For patients being placed on a breathing machine in an emergency department or an intensive care unit that is part of the RSI study:
- When the doctors feel ketamine would be best for a patient, the doctors give the patient ketamine.
- When the doctors feel that etomidate would be best for a patient, the doctors give the patient etomidate.
- When the doctors do not have a preference, the patient participates in the RSI study and assigned either ketamine or etomidate randomly. This means every patient has a fair and equal chance of getting either drug.
Remember: Both drugs are commonly used to place patients on breathing machines. Both are approved by the FDA for this use. Both are deemed safe and effective. Only when doctors do not have a preference does the RSI study choose the drug – and even then the doctors can always choose to use another drug to ensure the best care for the patient. During the placement of a breathing tube doctors and nurses are standing with the patient, closely watching to keep them safe, and preventing and treating possible problems.
Consent for Emergency Care
Placing seriously ill adults on a breathing machine is an emergency procedure. There is often no time for doctors to discuss the risks and benefits of the procedure or of the drug that will be used. Patients are often unconscious or too sick to make decisions. So, doctors go ahead with life-saving care without the patient’s okay (consent).
Consent for Research during Emergency Care
For these same reasons, it may not be possible for patients to give consent to take part in the RSI study. Important research to find the best emergency care, like the RSI study, can sometimes be done without getting patients’ okay (consent) ahead of time through a process called “Exception from Informed Consent” (EFIC). Studies done with EFIC are designed with input from patients, families, and community members and overseen by the FDA (FDA 21 CFR 50.24 ) and an independent ethics committee.
If you are seriously ill and are being placed on a breathing machine in an emergency department or intensive care unit taking part in the RSI study, your doctors feel that using either ketamine or etomidate would be safe and effective for you, AND there is not enough time to ask you or your family if you would like to take part in the RSI study:
- You would be enrolled in the study.
- You would receive whichever of the two drugs is randomly chosen by the study.
- A study team member would come to you (or your family) after the procedure and give you information about the study, answer your questions, and let you know how you could choose to stop taking part in the study.
Participating Sites
- Vanderbilt University Medical Center
- University of Colorado, Anschutz
- Denver Health Medical Center
- Wake Forest Atrium Health
- Hennepin County Medical Center
- University of Alabama, Birmingham
Frequently Asked Questions
What is a breathing machine?
Some seriously ill patients in the hospital need help to breathe. In this situation a breathing machine – also known as a “mechanical ventilator” – is used to help the lungs. A breathing machine helps move air in and out of the lungs to maintain proper levels of oxygen in the blood. To use a breathing machine, the doctors and nurses insert a tube through the mouth to reach the lungs. The procedure for inserting a tube into the mouth to reach the lungs is called “intubation”.
Who is eligible for the RSI study?
Adults who are receiving treatment in an emergency room or intensive care unit (ICU) whose doctors have determined that they need a breathing tube may be eligible for this study. Patients are eligible only if their doctors and nurses think that either of the study medications would be safe and effective for the patient. Patients are not eligible if they are allergic to one of the study medications. Patients can only participate if they are undergoing breathing tube placement at a hospital participating in the study. They cannot volunteer at any other time.
What is the difference between ketamine and etomidate?
You can probably imagine how hard it would be to have a tube put in your throat if you were awake. Ketamine and etomidate are both medicines that doctors give to make patients sleepy (sedate them) during the placement of a breathing tube. The medicines differ in how they make patients sleepy. Ketamine makes patients sleepy through one signal in the brain and etomidate uses a different signal in the brain. The medicines also differ in their possible side effects, as described below. Both medicines have been used during placement of a breathing tube every day in clinical care for decades.
What are the side effects of each medication?
When ketamine is used to sedate patients for the placement of a breathing tube, the most common side effects are altered mental status and nausea or vomiting. Uncommon but more severe potential side effects include serious problems with heart rate and blood pressure, allergic reactions, and buildup of pressure around the brain.
When etomidate is used to sedate patients for the placement of a breathing tube, the most common side effects are a sudden spasm of the muscles, low levels of stress hormones, and nausea or vomiting. Uncommon but more severe potential side effects include serious problems with heart rate and blood pressure, allergic reactions, and spasm of the vocal cord.
Why would a doctor choose one medication over the other?
For most patients, doctors do not have a reason to think that one medication would be better than the other. Rarely, a patient has an illness for which doctors know that one medicine would be better than the other. For example, for a patient with very high blood pressure, doctors may choose to use etomidate rather than ketamine because ketamine may increase blood pressure. When doctors do not have a reason to think that one medication would be better than the other, they may choose the medication that is most immediately available in their hospital, most common in their region of the country, or most often used by other doctors in their specialty. The purpose of the RSI trial is to provide information to doctors to ensure they know which medication is best for which patient.
Why did you choose to study ketamine versus etomidate?
Each year millions of patients undergo breathing tube placement in an emergency room or ICU. Because doctors believe that ketamine and etomidate are equally effective for breathing tube placement, some use ketamine, and some use etomidate. However, breathing tube placement can be dangerous, especially for seriously ill patients. It is important to know if ketamine or etomidate improves outcomes for some patients or all patients during placement of a breathing tube. The only way to figure out if ketamine or etomidate is better for some or all patients is to do a study like this one. We hope this study gives sick patients a better chance of surviving their illness and improves their long-term mental health.
When will you tell patients which medication they received?
The research team tells patients (and their families) which medicine the patient received shortly after the breathing tube is placed. In the RSI study, patients, families, doctors, nurses, and researchers all know which medicine the patients received throughout the study.
How are patients randomized to receive either ketamine or etomidate?
Research studies often use two groups of participants to compare treatment options. Because people are different in many ways, those groups need to have a similar mix of people. This is true for things like age and background. If the groups are too different, the research team might not be able to learn from the study. The best way to get a similar mix is to put patients in each group randomly. This is called randomization. Randomization means that each patient in the study has a fair and equal chance of receiving either medication. If a patient’s doctors and nurses think that either ketamine or etomidate would be safe and effective for the patient, the doctor lets the study decide which medication the patient will get.
Why are you sharing information about this trial with the community?
The goal of the RSI trial is to produce information that helps patients, families, doctors, and nurses choose the medicine that produces the best outcomes for patients receiving a breathing tube in the emergency room or ICU. Making sure that patients, families, and community members know about the study and its findings is important to achieving this goal. For that reason, patients, family members, and community members helped design the study, are helping to lead it, and will help us share the results of the study when it is completed-with patients who participated and with the community. We want people to be aware of the study, have an opportunity to ask questions and have their voices heard.
Patient Stakeholder Quotes

Eileen Rubin
Co-founder of the ARDS Foundation, has served as President and CEO for over two decades. An attorney by profession, Ms. Rubin experienced a life-altering diagnosis in her early 30’s that resulted in a lengthy ICU stay and long road to recovery. Today, she is a well- known advocate for patients and their families serving in a variety of roles to help educate medical professionals and to improve and inform research. Ms. Rubin stresses, “Including the patient and family perspective is critical in medical research to ensure studies are designed from beginning to end with the patient in mind and with objectives focused on concerns, issues and endpoints of importance not only to advance medical research but also to include priorities of patients.” She has served in an advisory capacity for numerous organizations including the American College of Chest Physicians, the Society of Critical Care Medicine and the American Thoracic Society. She was also the lead investigator for a PCORI Pipeline to Proposal Award. Ms. Rubin is a Patient Stakeholder for the RSI Trial.
Sherman Transou
was an active business owner but in 2015 his life was changed when he learned that a virus was attacking his heart. Five months later he joined the growing community of transplant recipients and has embraced this opportunity to inspire and educate others in his community. In addition to serving on the Board of Directors for HonorBridge, he is an active advocate and leadership coach. Mr. Transou uses his experience to help research teams effectively connect with patients and their families. Mr. Transou is the Patient Stakeholder for Atrium Health Wake Forest Baptist.


Barbara Gould
is a COVID intubation and liver transplant survivor and has personalexperience with post ICU syndrome and PTSD. As a retired social worker, Ms. Gouldunderstands the importance of patients’ physical and mental health and has used herexperience to platform the needs of patients and families. She shared, “I strongly believethat medical research saves lives and that patient representation in that process isessential.” Ms. Gould has spoken with the media about her hospitalization with COVID to raise awareness. She currently serves as the Patient Investigator for the University of Colorado Anschutz and the University of Colorado Denver.
Patrick Luther
brings a wealth of knowledge to the RSI team as someone who has experience in advising the research enterprise at large, clinical trials in particular, and as a critical care survivor and former paramedic. Professionally, he works in nonprofit public health spaces developing and managing programs and community engaged research projects that address the needs of our most vulnerable while building their agency to take their place within research to change and improve it. His experiences provide insight into patient communication and troubleshooting for trial implementation. Mr. Luther is the Patient Stakeholder for Vanderbilt University Medical Center.


Jasmine McIntosh
is a young adult cancer survivor who is an active advocate in the community. She is passionate about health inequities and supports research that is working to improve outcomes for all patients. Her background in systems and technology is an asset to the RSI Trial. “I believe clinical trial research is important because for me personally, as a two-time cancer survivor with a rare gene, research has allowed me to be on the receiving end of innovative care. I am grateful for that access as not everyone has that same opportunity. Research helps to make it more accessible and to continue the work toward health equity.” Ms. McIntosh is the Patient Stakeholder for the University of Alabama, Birmingham.
Aida Strom
has served as an advocate in the American Indian community for over 19 years. She has spent her career building strong connections between the community, tribal government entities, hospital, and patients and their families. In addition to working with patients and researchers, she has extensive expertise in working with individuals and communities suffering from PTSD and has worked closely with numerous community organizations including the Minnesota Indian Women’s Sexual Assault Coalition. Ms. Strom’s broad experience with underrepresented communities and specific expertise advocating for them is an incredible asset to the RSI team. Ms. Strom serves as the Patient Stakeholder for the University of Minnesota Hennepin.

We want to know what you think about the RSI Trial
We want feedback from the community about research like this. Please share your opinions at: https://redcap.link/rsitrial
If you do not wish to participate in this study
If you would not want to participate in this research study can if you became severely ill and needed treatment with a breathing machine, contact us at Jonathan.D.Casey@vumc.org or 615-208-6139. We will send you a bracelet you can wear that will inform your medical team of your decision even if you are unconscious.
